Vanda Pharmaceuticals Inc. VNDA recently experienced a dip in its stock price after receiving a complete response letter from the FDA regarding its new drug application (NDA) for tradipitant, a potential treatment for gastroparesis.
The announcement led to a 6.1% decrease in the company’s shares on Sept. 19, highlighting the regulatory setback.
Gastroparesis, characterized by delayed gastric emptying, is a condition that significantly impairs the stomach’s ability to process food. Alarmingly, there has been no FDA-approved medication for this condition in more than four decades.
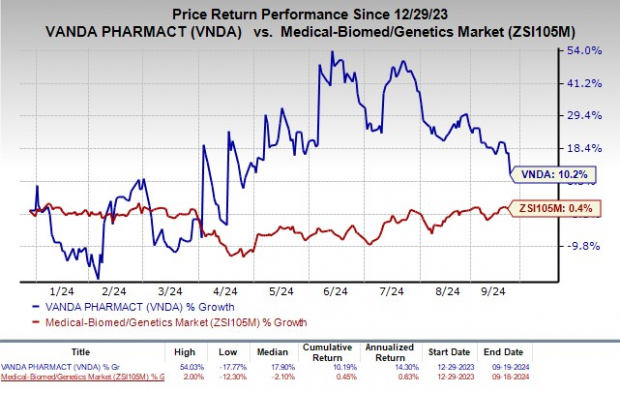
So far this year, Vanda’s shares have seen a 10.2% rise, outperforming the industry’s modest 0.4% growth.
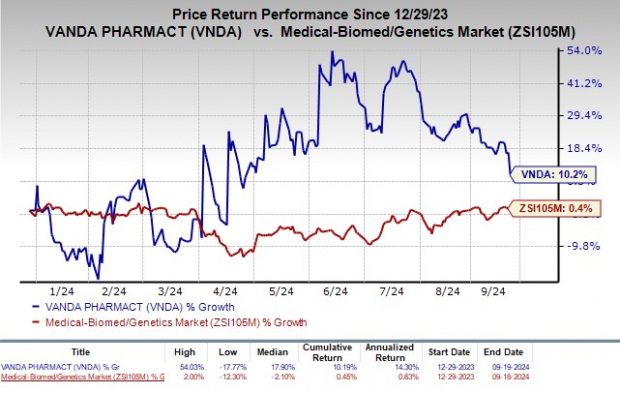
Image Source: Zacks Investment Research
Concerns Over FDA’s Response to VNDA’s Tradipitant
The FDA’s Complete Response Letter mandated that Vanda conduct additional studies on tradipitant, employing a methodology that contradicted expert advice in the field and lacked scientific rationale for treating the disease effectively.
Moreover, the FDA’s significant delay of over 185 days in making a decision failed to comply with the guidelines set forth by the Food Drug and Cosmetic Act (FDCA).
Under the FDCA, the FDA is obligated to review an NDA and issue either an approval or a potential hearing within 180 days of submission. Unfortunately, the FDA did not meet this deadline.
Vanda had repeatedly requested an advisory committee meeting with the FDA to discuss tradipitant’s NDA, but these requests were declined by the regulatory body.
In response, some patients who have benefited from tradipitant have lodged a Citizen Petition urging the FDA to approve the drug for treating gastroparesis.
VNDA’s Future Plans for Tradipitant
Despite the setback, Vanda remains steadfast in its pursuit of marketing approval for tradipitant to address gastroparesis symptoms. Additionally, the company is exploring the drug’s potential for preventing motion sickness-induced vomiting.
In May 2024, Vanda disclosed positive outcomes from a second phase III trial investigating tradipitant’s efficacy in combating vomiting due to motion sickness.
The company intends to submit another NDA for tradipitant to the FDA in late 2024, this time focusing on its application in motion sickness treatment.
Market Insights and Recommendations
Presently, Vanda holds a Zacks Rank of #4 (Sell). For investors seeking alternative options in the biotech sector, companies like Illumina, Inc. (ILMN), Krystal Biotech, Inc. (KRYS), and Fulcrum Therapeutics, Inc. (FULC) present favorable investment prospects with a Zacks Rank of #1 (Strong Buy).
Recent estimates for Illumina’s 2024 earnings have been revised upward, demonstrating a positive outlook for the company. Meanwhile, Krystal Biotech and Fulcrum Therapeutics have witnessed favorable developments in their respective earnings projections.
Despite the setback faced by Vanda Pharmaceuticals, the biotech landscape offers promising investment opportunities for discerning investors.